There are multiple ways to interpret blood gases. This is one step-wise approach:
Step 1: Look at the patient
Think about why the gas has been taken and what it might show given the clinical context.
Step 2: Oxygenation
This is important to consider first, as this is the most urgent to correct if grossly abnormal.
It is important to look at the PaO2 relative to the inspired oxygen concentration. The expected PaO2 can be approximated by multiplying FiO2 (in %) x 5. For example, 21% x 5 = 100mmHg.
The A-a gradient can be calculated (PAO2 – PaO2). If normal this suggests hypoventilation (or low FiO2). If raised then this suggests V/Q mismatch, shunt or increased O2 extraction.
Normal A-a gradient is [Age/4] + 4.
Step 3: Acid-base status
- pH
- If < 7.35, acidaemia present
- If > 7.45, alkalaemia present
- If between 7.35 and 7.45 it is possible to have a fully compensated metabolic or respiratory derangement.
- PaCO2 and HCO3– / Base Excess (BE)
- Looking at these values will help determine the primary cause of derangement:
- If acidaemic and CO2 high = respiratory acidosis
- If alkalaemic and CO2 low = respiratory alkalosis
- If acidaemic and HCO3– low (or BE more negative than -2) = metabolic acidosis
- If alkalaemic and HCO3– high = metabolic alkalosis
- Mixed pictures are possible e.g. acidaemia, high CO2 and low HCO3–.
- Looking at these values will help determine the primary cause of derangement:
- Compensation
- A respiratory acidosis should drive a metabolic compensation – an increase in HCO3–and vice versa.
- A metabolic acidosis should drive a respiratory compensation – a decrease in CO2, via an increased RR (this is why a tachypnoeic patient may have a metabolic acidosis). This is much faster than metabolic compensation. It is important to note that a patient can only reduce their CO2 to a certain limit.
- Broadly:
- if no compensation has occurred, then either CO2 or HCO3– (depending on the primary derangement is) will remain normal with an abnormal pH
- e.g. in a metabolic acidosis CO2 remains normal and pH < 7.35
- if some partial compensation has occurred then the pH won’t have normalised, but there will be some compensatory change from the normal CO2 or HCO3– values.
- e.g. in a metabolic acidosis, CO2 will be lower than normal but the pH will still be < 7.35
- if fully compensated state then pH near normal and CO2 or HCO3– abnormal.
- e.g. in a metabolic acidosis, CO2 will be low and pH between 7.35 and 7.35
- the pH may not be fully normal due to the maximal constraints of the compensatory response
- if no compensation has occurred, then either CO2 or HCO3– (depending on the primary derangement is) will remain normal with an abnormal pH
- Is the compensation sufficient?
- There are some rules that can be used to work out if the degree of compensation is what should be expected.
- Metabolic Acidosis (Winter’s Rule): Expected PaCO2 = (1.5 x HCO3–) + 8 (+/- 2)
- For example, if HCO3– is 12, then expected PaCO2 is 24-28. If the PaCO2 is 45 then a concomitant respiratory acidosis is also present.
- Metabolic Alkalosis: Expected PaCO2 = (0.7 x HCO3–) + 20 (+/-2)
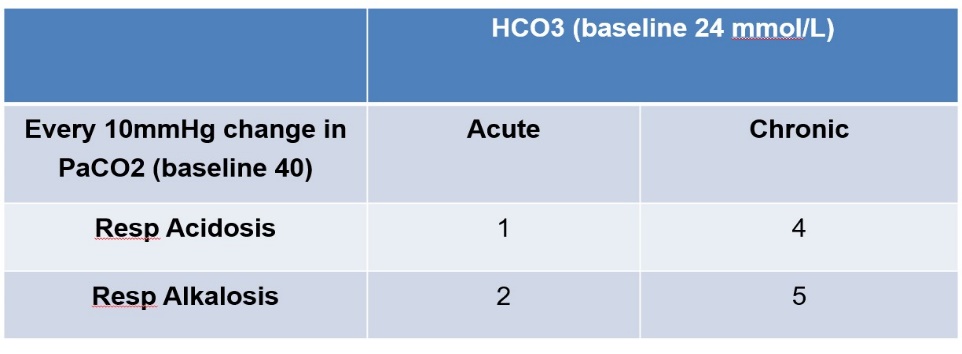
- Respiratory Acidosis and Alkalosis – “1,2,4,5 Rule”
- This will approximate how much the HCO3– should change for a given change in PaCO2. The table below shows how much you would expect the HCO3– to increase or decrease for every 10mmHg change in PaCO2 .
- For example in a chronic, well COPD patient with a HCO3– of 32 mmol/L (4 mmol/L above baseline for every 10 mmHg increase in PaCO2 ), a PaCO2 of 60mmHg would roughly be at their baseline. If their PaCO2 was acutely raised to 60mHg then you would expect the HCO3– to only be ~ 26mmHg (1 mmol/L above baseline for very 10 mmHg increase in PaCO2 ).
Step 4: Everything Else
Other important information on a gas that should be considered include:
- Electrolytes
- Haemoglobin
- Lactate
- Anion Gap
- Important in working out aetiology of metabolic acidosis
- Na+ – (Cl– + HCO3–)
- Normal is 4 – 12 mmol/L
- Can classify into normal anion gap metabolic acidosis (NAGMA) versus raised anion gap metabolic acidosis (RAGMA)
- More complex calculations such as osmolar gap and delta ratio can be carried out.
References and Further Reading
Author: George Walker
