This page will briefly touch on some of the common haematological issues encountered in ICU, namely transfusion and anticoagulation.
Transfusion
Given the frequency with which critically ill patients develop anaemia, thrombocytopenia or a coagulopathy, the use of blood products in ICU is extremely common. If considering correcting these abnormalities it is always important to consider the risks and benefits of doing so, as transfusion of blood products can result in patient harm. This chapter is going to consider blood transfusion in both the non-bleeding and bleeding patients.
Practices vary widely both within and between critical care units, and as ever, local guidelines and senior advice should be followed. The evidence below relates to the National Blood Authority Australia Critical Care guidelines:
The Non-Bleeding Patient
- Transfusion practice should be restrictive.
- Consensus opinion suggests that:
- A patient’s clinical state is equally as important as the haemoglobin (Hb) concentration (ie. are they symptomatic with a given Hb concentration?)
- Hb < 70 will likely require a transfusion.
- Hb > 90 will not likely require a blood transfusion.
- Some will argue for an Hb 80 – 100 for patients with acute coronary syndrome.
- Platelets only required if < 20 (or < 50 if undergoing an invasive procedure, and these should be given peri-procedurally).
- Fresh Frozen Plasma (FFP) and cryoprecipitate shouldn’t routinely be used in non-bleeding patients.
Transfusion is a widely studied topic in critical care. Below are some of the recent trials investigating optimal haemoglobin targets and best use of packed red blood cells:
- TRICC – A restrictive strategy (Hb < 70) is at least as effective, if not superior, to liberal blood transfusion targets (Hb < 100).
- TRISS – no difference in mortality or ischaemic events when using Hb < 70 vs. Hb < 90 as a blood transfusion threshold for patients with septic shock.
- TRANSFUSE – no benefit of using the freshest blood versus the oldest (< 42 days).
- TRICS III – A restrictive (Hb < 75) is non-inferior to a liberal strategy (Hb < 95) in post cardiac surgical patients
Massive Transfusion Protocol (MTP)
Massive transfusion is defined as:
- 100% replacement of circulating volume in 24 hours
- 50% replacement of circulating volume in 4 hours
The circulating volume is approximately 70mls/kg.
The MTP should be activated for patients that are bleeding and expected to meet the above definitions. Not only will the activation of a MTP provide urgent blood products, an automatic notification to the on-call haematologist and the laboratory occurs. This enables rapid prioritisation of samples and provision of clinical advice. Often designated staff to transport samples and blood products to and from the laboratory (“runners”) will also be allocated.
In the empiric treatment of major haemorrhage products are usually transfused in a 1-2 Packed Red Blood Cells (PRBC) : 1 FFP : 1 Platelet ratios. This is because sole transfusion of PRBC will worsen coagulopathy, as they contain no clotting factors. The PROPPR trial showed no mortality benefit of 1:1:1 vs 2:1:1 transfusion ratios.
Priorities in the critically unwell bleeding patient include:
- Appropriate IV access: ideally the shortest and widest device possible which results in increased flow. CVC lumens are not good for rapid transfusion. Ideally a 14 or 16G cannula, RIC line or MAC sheath are best.
- “Turn off the Tap”: arranging attempts to stop the bleeding: this may require surgeons, gastroenterologists or interventional radiologists.
- Avoidance of the “lethal triad” – acidosis, hypothermia and coagulopathy.
- Transfuse blood and blood products and avoid fluids – these will alter acid-base, temperature and have no oxygen carrying capacity or clotting factors.
- Regular monitoring including temperature, ABGs, FBE and either laboratory or point of care coagulation tests. Key aims include:
- Temperature > 35 ˚C (and giving warmed blood products).
- pH > 7.2
- PT < 1.5x normal, INR < 1.5
- Fibrinogen > 1 g/L
- Platelets > 50
- Ionised calcium (iCa) > 1
- Remember in the acute setting haemoglobin may be normal. Clinical and biochemical parameters (for example, drain output, blood pressure, lactate or increasingly negative base excess) are important.
- Consideration of tranexamic acid and reversal of any anticoagulants.
Thromboelastometry
Thromboelastometry is a point-of-care measurement of a patient’s coagulation state. There are two main tests in use – TEG and ROTEM. Simply, a pin is suspended in a sample of whole blood; as the blood clots fibrin strands develop between the pin and the blood so more torque is required for either the cup (TEG) or pin to be rotated (ROTEM). A normal ROTEM tracing (“a temogram” is given below, and this depicts the amount of torque required vs. time.
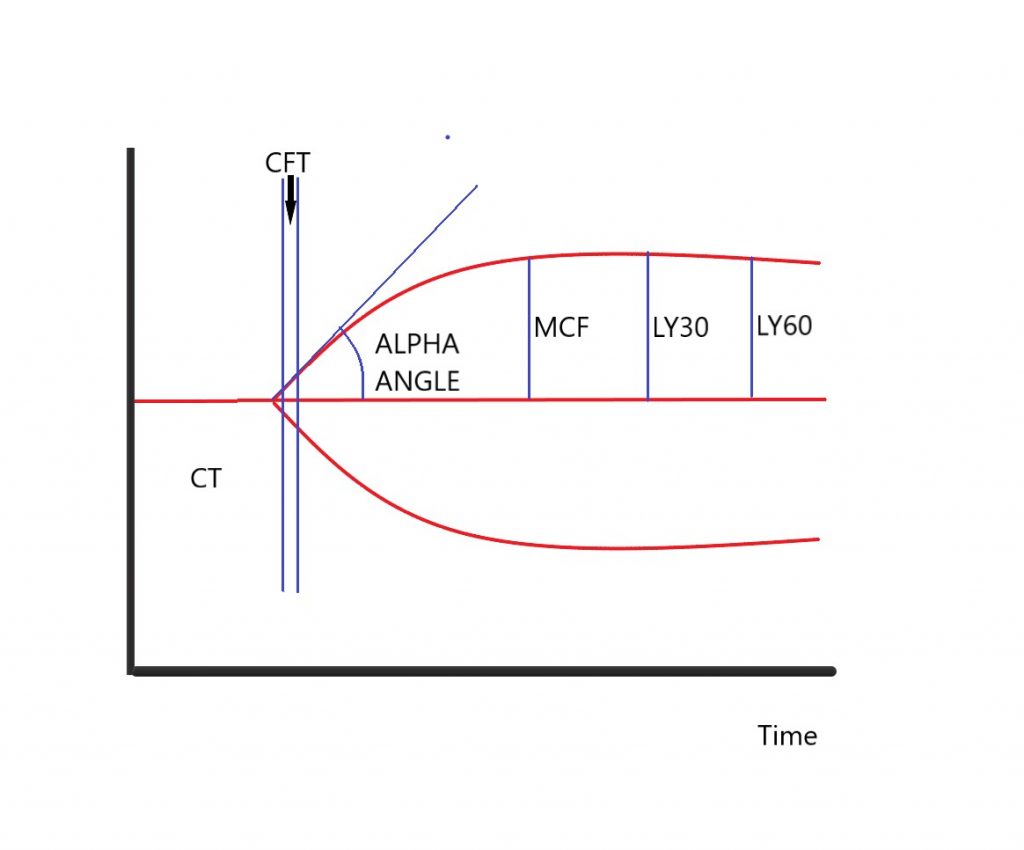
CT (R): Clotting Time – this is the time taken to 2mm amplitude on the trace
CFT (K): Clot Formation Time – this is the time taken from 2mm to 20mm on the trace
Alpha Angle – the angle of the tangent at CT
MCF (MA): Maximal Clot Firmness
LY30 (CL30): Lysis 30 – reduction in MCF at 30- and 60-minutes post CT
LY60 (CL60): Lysis 60
TEG tracings are similar to ROTEM but have slightly different nomenclature (given in brackets above).
How can we use this trace?
These traces are produced and displayed in real time, thus allowing for rapid identification of clotting deficiencies. If used then your local hospital will often have an algorithm in which how to interpret the trace and replace necessary blood products. An example of a MTP algorithm with ROTEM is the Sir Charles Gairdner ED MTP.
Briefly:
- CT is dependent on clotting factors (consider FFP if prolonged)
- The CFT and Alpha Angle dependent on fibrinogen (consider cryoprecipitate or fibrinogen concentrate if prolonged or shallow)
- MCF is dependent on fibrinogen and platelets
- The lysis phase reflects the fibrinolytic phase of coagulation and can be useful in hyperfibrinolysis (consider tranexamic acid if significant lysis occurring)
- Measurements can be made at 5 and 10 minutes (A5 and A10) and these have been shown to reflect the MCF value.
- Multiple assays are available and often run simultaneously. In the case of ROTEM these include:
- EXTEM – overview of extrinsic pathway.
- INTEM – overview of intrinsic pathway.
- HEPTEM – heparinase added thus negating heparin effect; useful in situations like cardiopulmonary bypass and ECMO where there is often circulating heparin and high risks of bleeding.
- FIBTEM – eliminates platelets (cytochalasin D added which inhibits platelets) thus provides a much better idea of the effect of fibrinogen.
- APTEM – plasmin inhibitors added (e.g. Tranexamic Acid) and can diagnose hyperfibrinolysis.
The high-quality evidence for ROTEM and TEG mainly exists in the cardiac surgery and trauma populations. Benefits suggested include reduced transfusion requirements (in particular FFP, platelets and packed red blood cells), faster availability of results, and cost.
Transfusion Related Harm
The risks of blood transfusion are important to know and understand, as this enables an ability to consent patients appropriately (consent is an important requirement of transfusion practice).
Immune Mediated
- Febrile, Non Haemolytic reaction (1:100)
- Urticaria (1:100)
- Anaphylaxis
- Haemolytic – Acute or Delayed:
- ABO incompatibility – rapid intravascular haemolysis
- Transfusion Related Acute Lung Injury (TRALI) – activation of pulmonary neutrophils resulting in non-cardiogenic pulmonary oedema. FFP has commonly been implicated as a trigger.
- Post Transfusion Purpura
- Transfusion Associated Graft vs Host Disease
Non immune mediated
- Transfusion Associated Circulatory Overload (TACO)
- Non-immune mediated haemolysis
- Sepsis or transmission of blood borne viruses (increasingly rare nowadays but historically has been a method of transmission for multiple disease).
- Iron Overload
- In massive transfusion: hypocalcaemia, hyperkalaemia, hypothermia, citrate toxicity can all occur.
Storage Lesions
Stored blood develops physiological changes when compared to blood that is currently circulating in vivo. These progress with increasing age of the stored blood.
These include decreased 2,3 DPG (left shifted oxyhaemoglobin dissociation curve), haemolysis, hyperkalaemia, acidaemia, deformation to RBC shape which can impede microvascular flow when transfused and accumulation of proinflammatory cytokines.
Anticoagulation in ICU
VTE prophylaxis
VTE prophylaxis should be considered daily for every ICU patient and all patients should be on pharmacological prophylactic anticoagulation unless you can justify not starting it.
Non pharmacological measures are important to consider too (in particularly TED stockings or sequential compression devices).
Pharmacological Treatments:
- Low Molecular Weight Heparins (eg. Enoxaparin)
- Potentiates action of Anti-Thrombin III (ATIII) which increases inhibition of factor Xa (and IIa in 4:1 ratio).
- For VTE Prophylaxis: usually dosed at 40mg/day
- For “treatment” dose: 1mg/kg BD
- Renally eliminated so if impaired renal function will require dose reduction. Extremes of weight may also require dosing adjustment and local hospital VTE prophylaxis protocol should be consulted and may recommend treatment with a heparin infusion.
- May require anti-Xa level measurement – these should be measured 4 hours after the 2nd or 3rd dose. Low levels of Anti-Xa require LMWH dosage increases and vice versa.
- Heparin
- Also potentiates effect of ATIII but its larger size (compared to LMWH) results in an increased inhibition of IIa and Xa (1:1 ratio) thus can be monitored with a APTT levels.
- Not renally eliminated so often used in patients with reduced renal function (CrCl < 30).
- Heparin infusions have almost immediate onset and also have the ability to be quickly switched off. Therefore they can be a better option in patients at high risk of bleeding, or awaiting a procedure where it is important to minimise time with no anticoagulation (e.g. prosthetic mitral valve).
- Heparin infusions require a bolus and then regular monitoring with APTT to guide infusion rates. Each hospital will have a protocol with frequency of APTT measurement and rate adjustments depending on the value. An APTT of 60-80 is usually targeted.
- Increased risk of Heparin Induced Thrombocytopenia and Thrombosis (HITT), which can have significant morbidity and mortality.
- Reversed with protamine (can cause profound pulmonary hypertension and histamine release so needs to be given slowly).
- Others:
- Fondaparinux – Indirect Xa inhibitor (similar to LMWH but no IIa inhibition)
- Bivalirudin – Direct Xa inhibitor
Important considerations in ICU patients and VTE thromboprophylaxis:
- Post-surgical – usually at discretion of surgical team and should be asked daily about appropriateness if not prescribed following surgery.
- Coagulopathy – often not required, although in liver disease patients may be pro-thrombotic and as such may be used.
- Epidural insertion or removal – must wait for 12 hours following the procedure before prophylactic LMWH can be given or 24 hours if treatment dose LMWH is prescribed.
PE Thrombolysis
In ICU the most common requirement for thrombolysis will be massive PE. It is important to distinguish between massive and sub-massive PE
A massive PE is an acute PE with haemodynamic instability; in sub-massive PE there is no haemodynamic instability however RV dysfunction or myocardial necrosis is present.
Systemic thrombolysis is a common treatment for massive PE, especially without the provision of catheter directed thrombolysis (or mechanical thrombectomy).
Much debate surrounds thrombolysis in sub-massive PE as it still has a significant morbidity and mortality.
Classically the dose is 10mg bolus of alteplase, followed by a 90mg infusion over 2 hours. CPR if initiated should also be prolonged.
All these patients should be discussed with your haemostasis and thrombosis team.
References and Further Reading
National Blood Authority Patent Blood Management Critical Care Guidelines
Australian Red Cross: Blood Products and Transfusion for Health Professionals
Life in the Fast Lane: VTE Prophylaxis
Author: George Walker
